QMS Feature
Highlights
Highlights
Built for teams that need results, not paperwork.
Easy13485 combines pre-configured processes, clear ownership, and built-in training into a QMS that actually works in startup reality.
Easy13485 is instantly ready, scalable, and aligned with ISO 13485 & MDR — so you can implement a working QMS in one day, not one year.

Easy13485 combines pre-configured processes, clear ownership, and built-in training into a QMS that actually works in startup reality.
No blank templates. All processes aligned with ISO 13485, MDR, ISO 14971, IEC 62304 and more. Define your scope and go live.
Forget multi-month rollouts. With Easy13485, you import, assign roles, and launch your QMS in 24 hours.
Built around predefined roles and responsibilities. Works with small or cross-functional startup teams.
Modular process building blocks your team actually uses – not just static PDFs or templates.
Directly linked to learning paths. Each process has built-in training assignments to keep your team qualified and audit-ready.
Easy13485 integrates ISO, MDR and IEC requirements into one practical Quality Management System: processes, templates, roles, and expert support — continuously maintained and structured for audits.
What you get
Your compliant foundation: QMS, MDR documentation, and risk management.
Software standards embedded into development and traceability.
Hardware, usability and verification steps guided via stage gates.
Clinical evaluation aligned with MDR and established guidance.
After launch: PMS, vigilance, complaints, CAPA, recall strategy.
IPP acts as regulatory consultant and confirms that medical device manufacturers committing to the Easy13485 Quality Management Service operate a complete and effective Quality Management System according to ISO 13485. The system is audited at installation and at regular intervals as required by relevant standards.
Our QMS isn’t flashy – and that’s the point. It’s built to pass audits, not beauty contests. 100% structured, tested, and actually usable. Still better than drowning in spreadsheets.
The EASY13485 QMS-Service gives you a fully structured, startup-optimized quality management system that’s pre-configured, pre-filled and audit-ready – with no extra burden on your team.
You don’t need to customize endless templates or “translate” standards into your workflow. With EASY13485, you’re up and running in a day – not in 12 months.
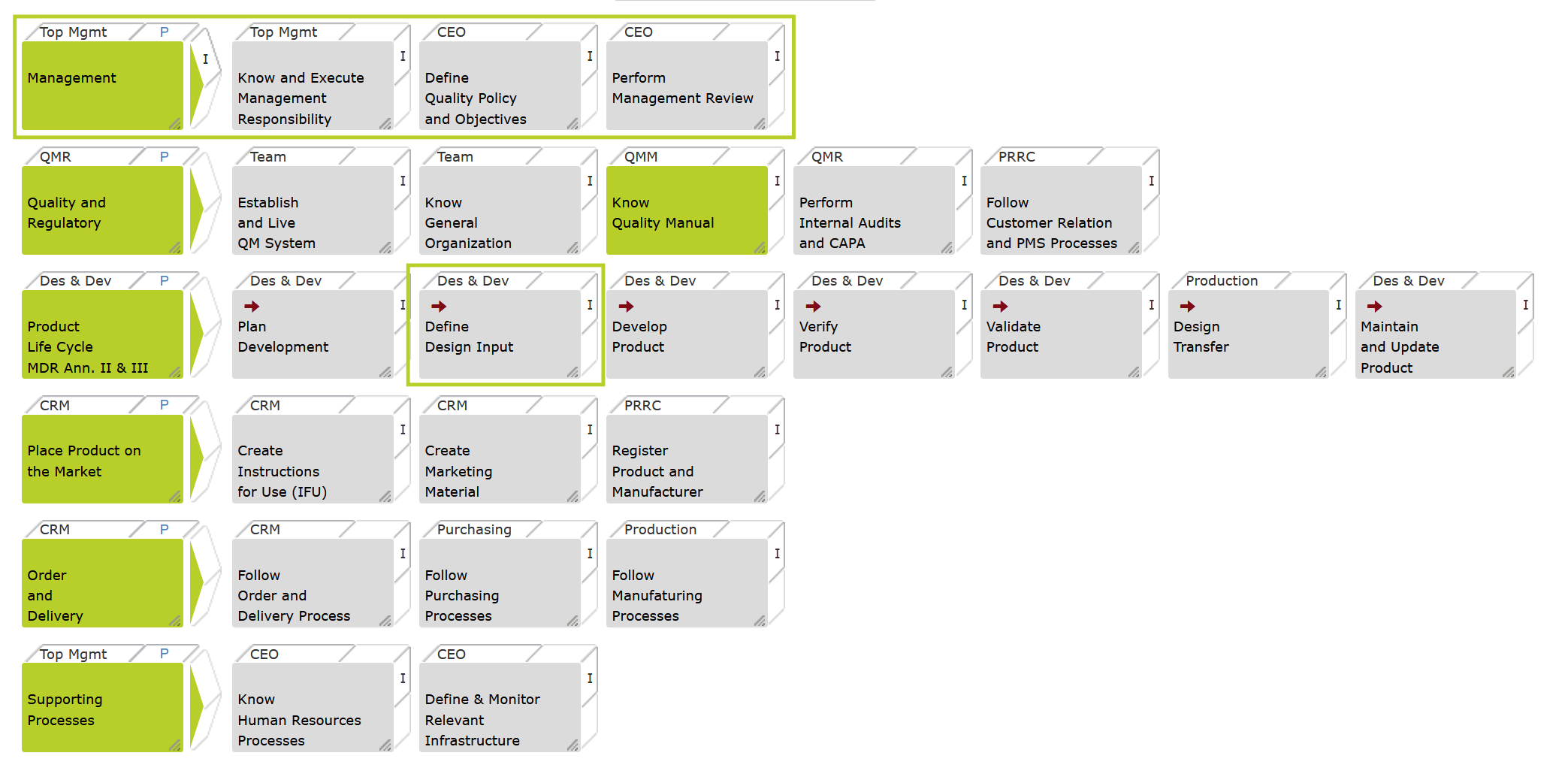
The core of EASY13485+ is a fully mapped ISO 13485 system, extended for EU and US regulatory expectations.
This means: your QMS is audit-ready, traceable, and credible for investors from day one.
EASY13485+ already maps the core requirements used by multiple international regulators.
This means: entering new markets doesn’t require reworking your entire quality system.
Beyond classic QMS, EASY13485+ integrates governance standards that are becoming mandatory for enterprise customers and AI-based products.
This means: fewer blockers in security reviews, AI assessments, and enterprise procurement.
Easy13485 delivers out-of-the-box speed with the flexibility to match each team’s unique setup.
Built to develop high potentials, the Academy turns training into a strategic growth tool.
Consultants are used strategically, not permanently.
Answers in seconds, so teams stay in flow and budgets stay lean.
Confidence on audit day – fewer findings, fewer costly loops.
Start smart. Choose how you want to begin.
For startups, we offer a simple entry into professional compliance and quality management.
Build the right foundation and get rewarded.
Start immediately with tailored expert support.
